Pharmaceutical industry
Pharmaceutical quality assurance and quality control, GMP and GLP testing laboratories.
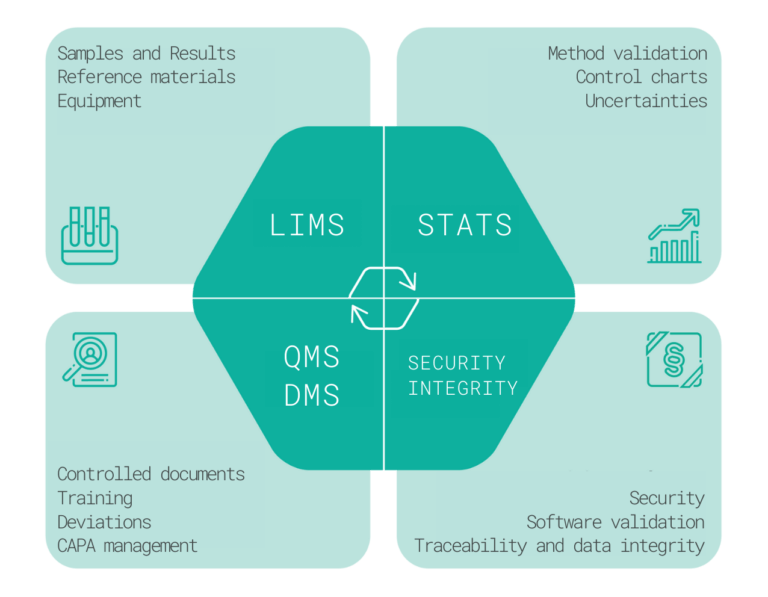
Characteristics and functions
EffiChem was developed in compliance with the harmonized GMP requirements for US, EU, JP, used worldwide, including US FDA 21 CFR Part 210 and 211, Volume 4 of EU GMP, Supplement 11 (Annex 11), 21 CFR Part 11 and GAMP 5 for validation of computerized systems.
LIMS is used to manage the lab processes and to ensure the related quality records. The basic process is the testing of samples/batches, recording test results and comparing them with the specification limits. This process consists of a sample receipt, sample logging, testing and results entry, review, approval, and reporting. This is based on previously created specifications and by development and validation of test methods, which are also handled and evaluated through the LIMS system. The same applies to equipment and calibration management, to reference materials certification and expiry monitoring, and to the management of chemicals, solutions, chromatographic columns, and auxiliary materials.
The LIMS module also enables effective planning, recording and evaluation of stability studies, recording of environmental monitoring data, and data from supporting systems. It can work with barcodes or QR codes and supports managerial reporting.
We are currently developing the connection of LIMS with different types of equipment and ERP systems.
The QMS modules can be used for record keeping and quality management of either the testing laboratory or the entire pharmaceutical company including recording and evaluation of deviations, change management, audits and observations, CAPA management (corrective and preventive actions, investigation of Out-of-Specification (OOS) and Out-of-trend (OOT) results, complaint handling, and risk management.
Workflows are configured according to your requirements and processes. Workflows can be enhanced with email notifications, task/action management, escalation rules, and other general functionalities, including managerial reporting.
Document management and training is an integral part of the entire Quality Management System. It can include the complete process from document creation, review, and approval, to activation/release, periodic revisions, archiving, or document cancellation.
Training management is linked to the documentation, starting with the creation of Tests, test questions, and the definition of correct answers, the ability to record and evaluate user responses, quantify the test score, initiate re-testing, monitor training deadlines, document complete and incomplete training, and remind and escalate missing training.
For Document Management and Training, workflows can be configured according to customer requirements and the desired process. Workflows can be enhanced with email notifications, task/action management, escalation rules, and other general functionalities, including managerial reporting.
Statistical Data Evaluation can be done either in the Methods module in LIMS, or in Samples, or configured individually. The assessment typically includes the evaluation of method validation data, estimation of uncertainties, development and use of control charts, creation and use of calibration models, and evaluation of inter-laboratory studies.
When there are multiple options for statistical evaluation of data, all options are included in the software, e.g. for a correct evaluation of Accuracy, options include a t-test and a statistical regression evaluation; for linearity, there is the correlation coefficient calculation, ANOVA or Sign test. The output of statistical data evaluation is a numerical result, a graph, and an automatically generated report.
Customer project support
EffiValidation 4.0



